Brain
The brain has developed to be one of our bigger research areas with multiple different projects and collaborations. Firstly, we introduce our research on the neurovascular coupling (NVC) – which is where we started off. Secondly, we also have projects in brain metabolism. Thereafter, we have branched further to do projects regarding ion channel kinetics, modelling of hormonal control, sociopsychological modelling, thought process modelling, and recently started project regarding the electrophysiological signaling of neurons.
- Biological mechanisms behind fMRI measurements
- Brain metabolism
- Ion channels kinetics
- Vulnerability, cortisol and sociopsychological modelling
- Hormonal regulation by the brain
- Thought processes
Biological mechanisms behind fMRI measurements
Functional magnetic resonance imaging (fMRI) is a commonly used approach for measuring brain activity. However, the measured fMRI dose not measure neuronal activity directly but rather a complex interplay between the blood flow, blood volume and the metabolism in the measured region. When a region of the brain is activated by external stimulus these components give rise to a hemodynamic response called the blood-oxygen-level dependent (BOLD) response. The BOLD- response is caused by a series of vasodilating and vasoconstricting reactions caused by neuronal activity. The mechanisms that the neuronal cells use to influence the blood supply are not fully known, and therefore it is difficult to know the true neuronal signalling only from inspection of the fMRI signal. In this project we utilize mathematical modelling, based on physiological understanding of the neurovascular coupling (NVC), to uncover the involved in the hemodynamic response cause by neuronal activation. Our models can accurately describe and predict the measured signals form a wide range of different aspects of the NVC, from visual stimulation in humans to optogenetic and sensory stimulation in mice. The results form out projects are providing the mechanistical insight necessary to bridge the world of intracellular neurobiology with the world of clinical radiology.
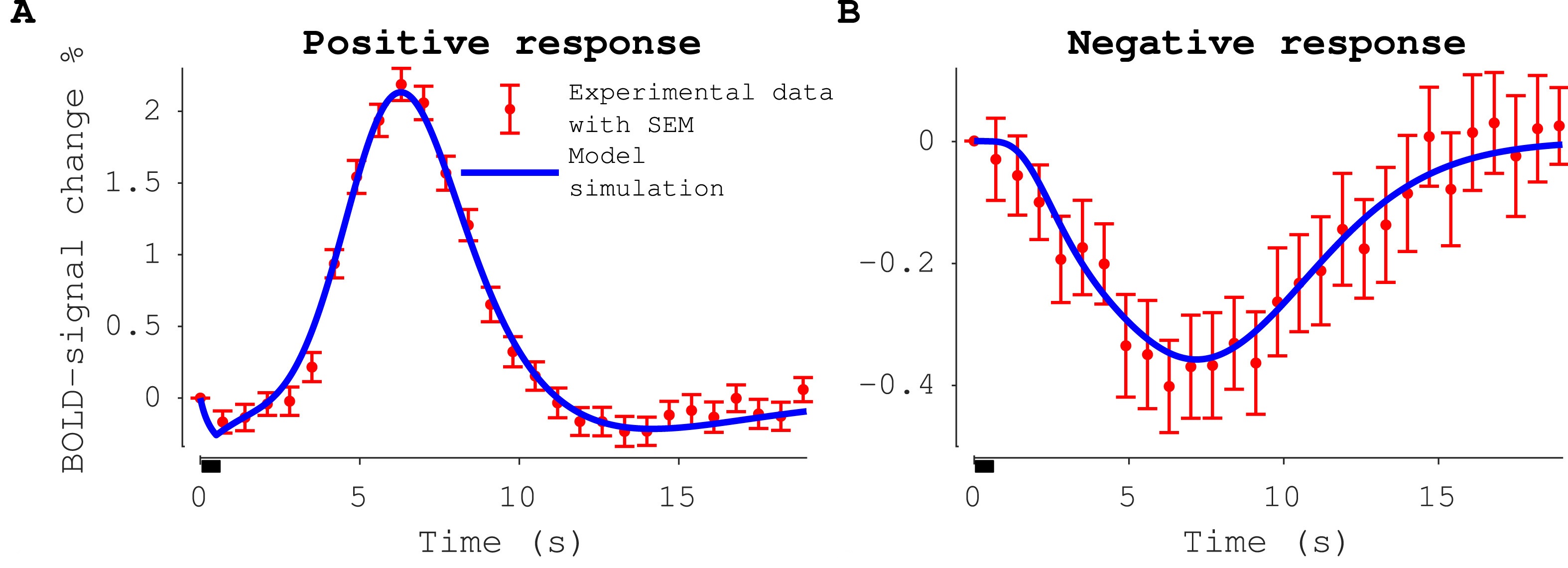
Our Collaboration partners for this projec are:
- Maria Engström, who is an expert on fMRI.
- Fredrik Elinder, who also is an expert on cell biology and electrophysiology.
Some key publications are
- Sten et al 2021, bioRxiv
- Sten et al 2020, NeuroImage
- Sten et al 2017, NeuroImage
- Lundengård et al 2016, PLOS computational biology
Brain metabolism
When it comes to metabolism, the brain is the most metabolic active organ being responsible for 20-25% of the body’s overall energy consumption. The brain’s main source of energy comes from oxidation of glucose. Therefore, the brain requires a continuous supply of glucose and oxygen and while the brain can metabolise stored glycogen to produce glucose, part of the required glucose and all of the oxygen is delivered via the blood. For these reasons, the cerebral metabolic glucose consumption is directly linked to the regional cerebral blood flow. The regional cerebral blood flow is, in turn, tightly coupled to the neuronal activity. An increase in neuronal activity will lead to an increased regional cerebral blood flow, which provides an increased supply of glucose. These couplings between the neuronal, hemodynamic, and metabolic activity is commonly known as the neurovascular coupling (NVC) and the neurometabolic coupling (NMC).
More specifically, the NVC describes how neuronal activity affects cerebral blood volume (CBV) and cerebral blood flow (CBF), while the NMC describes how neuronal activity affects the cerebral metabolic activity such as the cerebral metabolic rate of oxygen (CMRO2). The NVC is a cornerstone of functional magnetic imaging (fMRI), which is a key method for understanding how different stimuli affect regional neuronal activity. Data from fMRI is based on analysis of a blood-oxygenation-level-dependent (BOLD) signal. The BOLD signal is governed by the regional balance between the hemodynamic and metabolic activity i.e., by the balance between oxygen supply and oxygen consumption. Both the supply and the demand of oxygen are regulated by the neuronal activity. This connection between oxygen regulation and neuronal activity is the basis for why the BOLD signal is used as proxy for the neuronal activity. However, using the BOLD signal to draw correct conclusions is not straightforward since the crosstalk between hemodynamic, metabolic, and neuronal activity is nonlinear, time-varying, and partially unknown. One approach to deal with this complexity is mathematical modelling. We have developed a minimal mechanistic model that can describe the metabolic response to visual stimuli. The model is trained to describe experimental data for the relative change in metabolic concentrations of several metabolites in the visual cortex during stimulation. The model is also validated against independent validation data, that was not used for model training. Finally, we also connect this metabolic model to a detailed mechanistic model of the neurovascular coupling. Showing that the model can describe both the metabolic response and a neurovascular response simultaneously.
Some key publications are
Ion chanel kinectics
Voltage-gated ion channels generate and shape nervous and cardiac action potentials. These ion channels are the fundamental basis for neuronal electrophysiological activity and to quantitatively describe and predict the role of disease-causing mutations and the effect of pharmaceuticals, detailed models for ion channel kinetics are needed. Some models exist for these purposes, not least of all the equations developed by Alan Hodgkin, Andrew Huxley, and Bernhard Frankenhaeuser, these equations are the gold standard for calculation action potentials but these models where developed in the 60’s and we have learned a lot more about the ion channels since then. For instance, there are aspects regarding the structure and the mechanisms of opening the ion channels that the Hodgkin-Huxley equations does not take into account, as well as the several steps involved in the channel activation. Furthermore, these models are often described in different formats which makes comparison between different model structures difficult. In a project we conduct together with Fredrik Elinder, we have developed a general and flexible model structure that can be adapted to describe different ion channels. Our results show that our new model structure can describe data as well as the Frankenhaeuser-Huxely model but with more physiologically interpretable parameters. Further our model structure can also describe some aspects of the ion channel kinetics that the Frankenhaeuser-Huxely model is not capable of describing.
- Fredrik Elinder, who is an expert on cell biology and electrophysiology.
Vulnerability, cortisol and sociopsychological modelling
When considering the status of a patient, there are important factors that determine the outcome, beyond the mere physiological factors. The difference between whether a patient has e.g. a low/high education, low/high income, a big/small social network, etc, makes a difference in several years of life expectancy, and heavily influences the risk of contracting e.g. a cardiovascular event. Those factors are social factors. There are other similar psychological factors, such as depression, degree of optimism, which have similar high influence on the health of a patient. Note that these sociopsychological factors have a strong predictive ability for the outcome of a patient, also when compensating for the purely physiological differences, such as blood pressure, weight, plaque, etc.
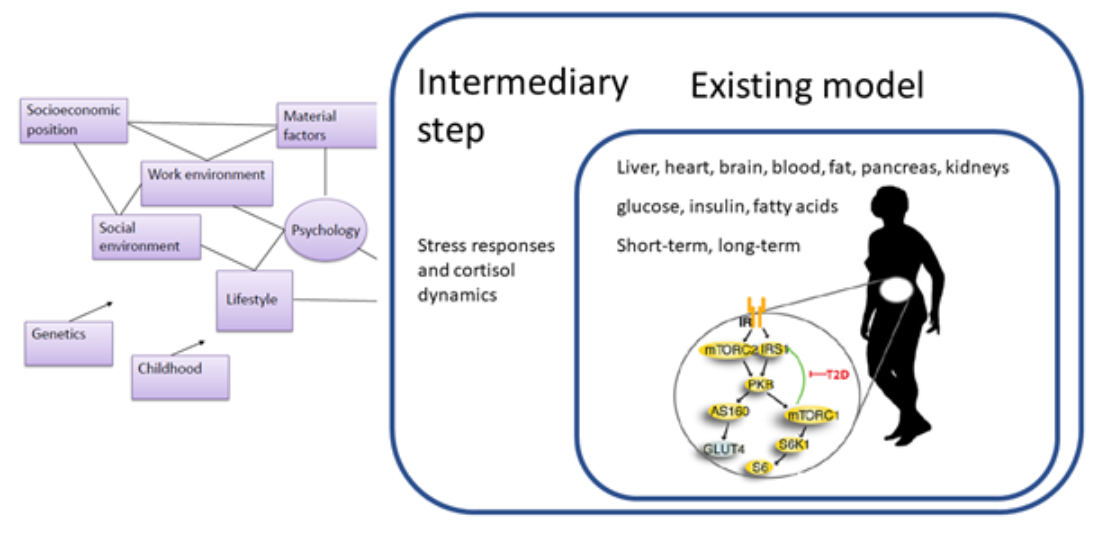
Because of this importance, we want to combine our models for the purely physiological processes, with corresponding interplay with sociopsychological factors. We do this by collaborating with social medicine MDs and professors such as Margareta Kristensson and Lena Jonasson, who have studied the cross-talk between these factors for many years. They contribute with the expertise, and with data from past and ongoing studies. To start with, we have constructed models for the cortisol dynamics in different patient groups, coming from different sociopsychological backgrounds. This has been done as student projects by Kajsa Tunegård, who now will continue to work with this also as a Ph.D. student.
Hormonal regulation by the brain
The brain is important also because it controls many central hormones. Apart from cortisol regulation, described above, we have also modelled other hormones, such as the T4 hormone, regulated by the hypothalamus, and produced by the Thyroid gland. Dysregulations of the Thyroid hormones T3 and T4 are common, and one can both have too high levels (hyperthyroidism) and too low levels (hypothyroidism). Both these disorders are treated, and the goal is to bring back the hormonal levels back to normal. This is in principle similar to the treatment of type 1 diabetes, where the body has lost its ability to accurately produce the hormone insulin, which thus is given as a treatment. However, unlike for type 1 diabetes, where there are relatively well-developed methods, and even semi-closed loop solutions, that allows for the detailed calculations of how much insulin one should give, it is hard for many patients to control their T3 and T4 levels. To move towards a possible solution to this, we have created an initial model for the daily variations of these thyroidal hormones (Fig 4A), which can describe data reasonably well (Fig 4B). This project was done as a B.Sc. project by Simon Andersson.
Thought processes
We are also doing a research project where we are modelling the dynamics of thoughts more directly. This is a new exciting collaboration between experimental psychologist Johan Willander, in Gävle University, social medicine MD and professor Margareta Kristensson, clinical psychologist and Ph.D. student Tomas Lindegaard in the group of Gerhard Andersson, and physicist and fMRI expert Maria Engström. During 2020, we will do a new clinical study, where we will use semantic analysis to quantify the verbal responses of different subject groups into time-series of thoughts: displaying time-series of their chosen topics, reasonings, emotions, etc. This quantification is done by Willander and colleagues. While doing these interviews, the subjects will also be monitored with respect to various physiological parameters, such as skin conductance, Near-Infrared Raman Spectroscopy, heart-rate, body and eye-movements, etc. We will then analyze these time-series using mechanistic, stochastic models, based on different mechanistic hypotheses for how thought movements typically happens. These mechanisms are based on literature and clinical experience of Lindegaard. The ultimate goal is to be able to not only test hypotheses regarding how thought dynamics are governed in different sociopsychological patient groups, but to be able to use the expanded digital twin technology as a diagnostic and pedagogical tool, when interacting with patients, to either boost their sociopsychological profile, or to treat different psychological disorders.
